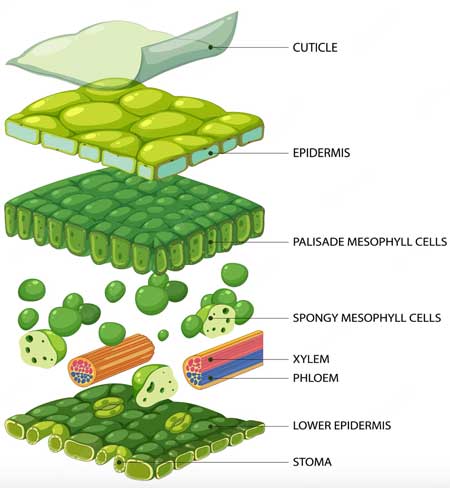
The research was carried out with the aim of investigating the effects of foliar spraying with micronutrient...
Blog categories
Latest posts
-
 Investigating the Effects of Foliar Spraying with Zinc Chemical Fertilizers and Zinc Nano-Chelate on the Quantitative Yield of Balango Shirazi plant (Lallemantia royleana Benth.)Read more
Investigating the Effects of Foliar Spraying with Zinc Chemical Fertilizers and Zinc Nano-Chelate on the Quantitative Yield of Balango Shirazi plant (Lallemantia royleana Benth.)Read more -
 Evaluation of Foliar Application with Nano Fertilizer (Super Micro Plus) in Different Times on Availability and Uptake of Some Micronutrients and Some Quality Properties of Rice2025-02-10Posted in: Articles and ResearchRead more
Evaluation of Foliar Application with Nano Fertilizer (Super Micro Plus) in Different Times on Availability and Uptake of Some Micronutrients and Some Quality Properties of Rice2025-02-10Posted in: Articles and ResearchRead moreThis field experiment was conducted in Najaf Ashraf province in Iraq in the summer of 2017 to investigate the effect...
-
 Reducing in vitro cultivation costs by replacing nano salts in order to commercialize this technique in Iran2025-02-10Posted in: Articles and ResearchRead more
Reducing in vitro cultivation costs by replacing nano salts in order to commercialize this technique in Iran2025-02-10Posted in: Articles and ResearchRead moreCultivation of some plants in vitro and field conditions is difficult and in some cases not cost-effective due to...
-
 The effect of salicylic acid priming and Chelat nanofertilizer (Parmis fertilizer 10) application on the biochemical characteristics of two chickpea (Cicer arietinum L.) cultivars under rainfed conditions2025-02-02Posted in: Articles and ResearchRead more
The effect of salicylic acid priming and Chelat nanofertilizer (Parmis fertilizer 10) application on the biochemical characteristics of two chickpea (Cicer arietinum L.) cultivars under rainfed conditions2025-02-02Posted in: Articles and ResearchRead moreSalicylic acid priming and Chelat nanofertilizer (Parmis fertilizer 10-specific fertilizer for beans) spraying can...
-
 The effect of nano-potassium fertilizer on growth factors, photosynthetic system and protein content of wheat2025-02-01Posted in: Articles and ResearchRead more
The effect of nano-potassium fertilizer on growth factors, photosynthetic system and protein content of wheat2025-02-01Posted in: Articles and ResearchRead moreThe use of nano-fertilizers to precisely control the release of nutrients can be an effective step towards achieving...